Biography
My expertise in biological products development and registration
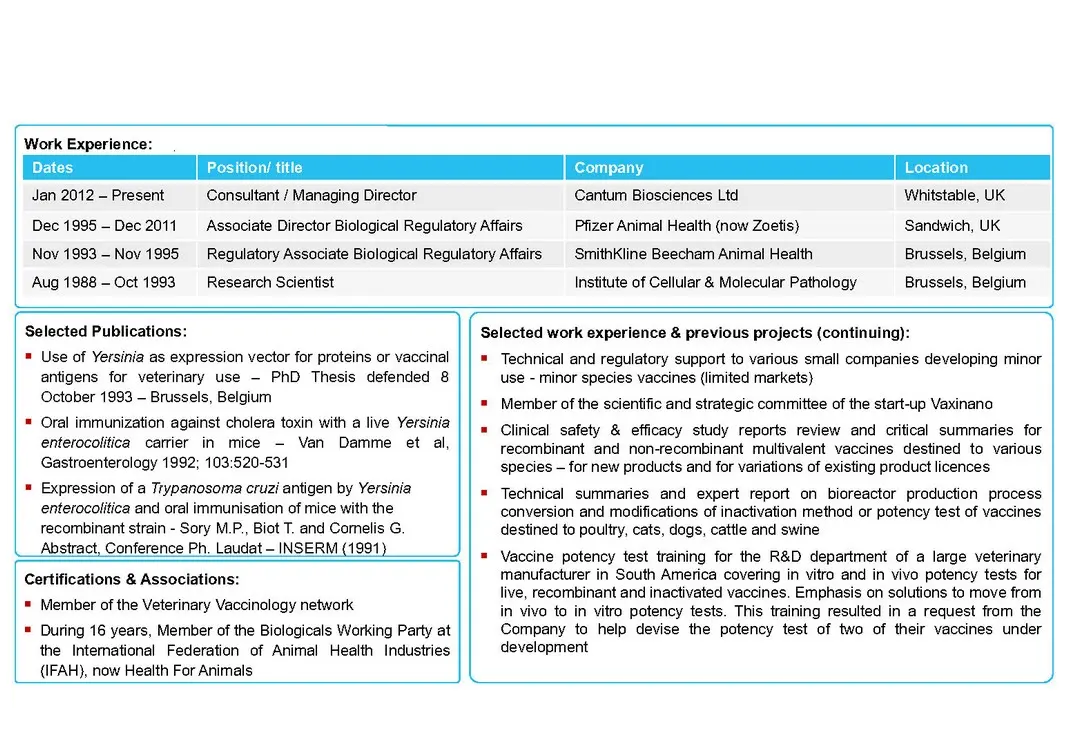
Cantum Biosciences Ltd
Thierry Biot, BA Hons, MSc, PhD, developed and registered veterinary vaccines during 20 years in the pharmaceutical industry. During this time, he delivered full technical development and registration guidance on veterinary vaccines, GMO and non-GMO, produced regulatory and technical gap analysis reviews, wrote critical reports and full registration dossiers, dialogued with multiples authorities, managed regulatory procedures, and answered their technical list of questions within tight deadlines.
Building up on his extended industry expertise, Thierry created Cantum Biosciences Ltd in 2011, whom he is the Director and unique Consultant. He maintains his Consultancy as a small independent structure without overheads to ensure his Client's access to the highest quality expertise, without intermediaries or work delegation.
Cantum Biosciences Ltd provides guidance on all aspects of the administrative, analytical and clinical development required for the registration of veterinary vaccine and biological products in EU such as production, quality control, assay development, validations, pre-clinical and clinical safety and efficacy or product claims wording. Thierry writes or reviews any CMC or clinical document for registration dossiers for first licence or for post-licensing variation. He helps you with any regulatory procedure, including pre-submissions requests such as Limited Market Authorisations and Scientific Advice.
Thierry is bilingual English & French, fluent in Portuguese and actively perfects his Spanish language skills.
Thierry is a molecular biologist by training. He holds a PhD in Medical Sciences, a Master Degree in Biomedical Technology and a Bachelor Degree in Biomedical Sciences, all granted by the University of Louvain in Belgium.
Expert advice and specialized reports
Who I Am